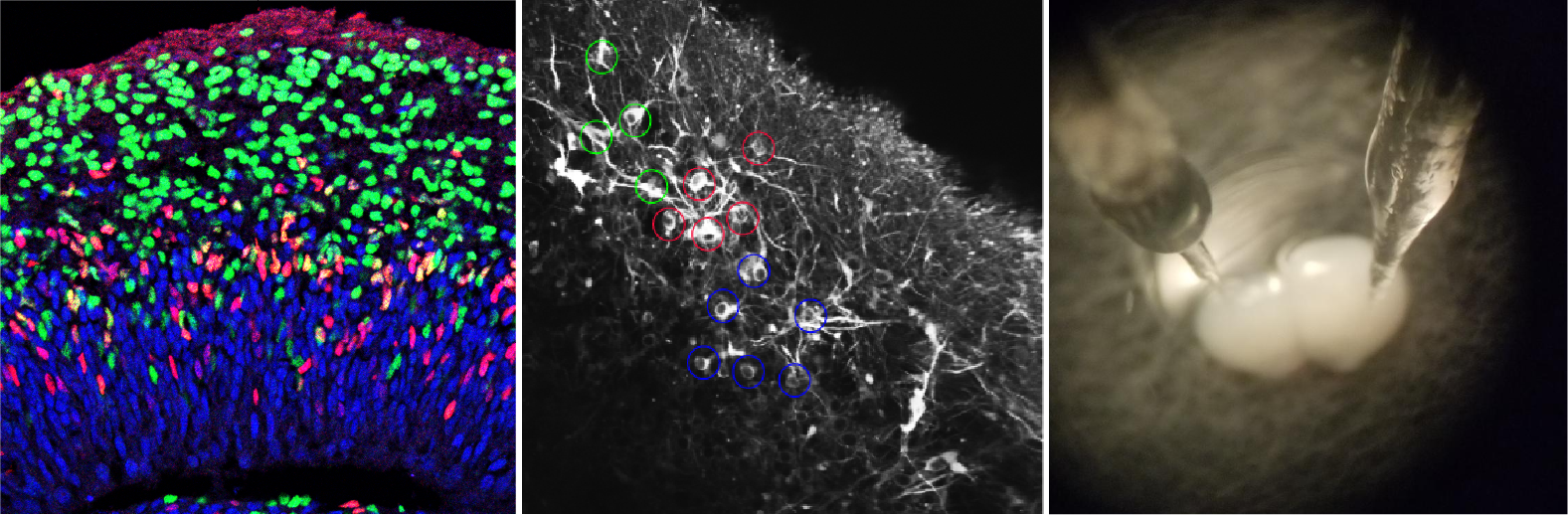
Philosophy:
Visit our primary lab website for the most updated information--> Neural Circuit Development and Dynamics Lab
People first. This is the guiding philosophy for our laboratory. Our greatest assets are the people in our lab, our collaborators, and the innovative ideas and enthusiasm they bring to our science. And, of course, the ultimate goal of our efforts is to help people; our patients with severe neurological disease such intractable seizures, epileptic encephalopathies, and autism, and the loved ones caring for them.
Scientific Overview:
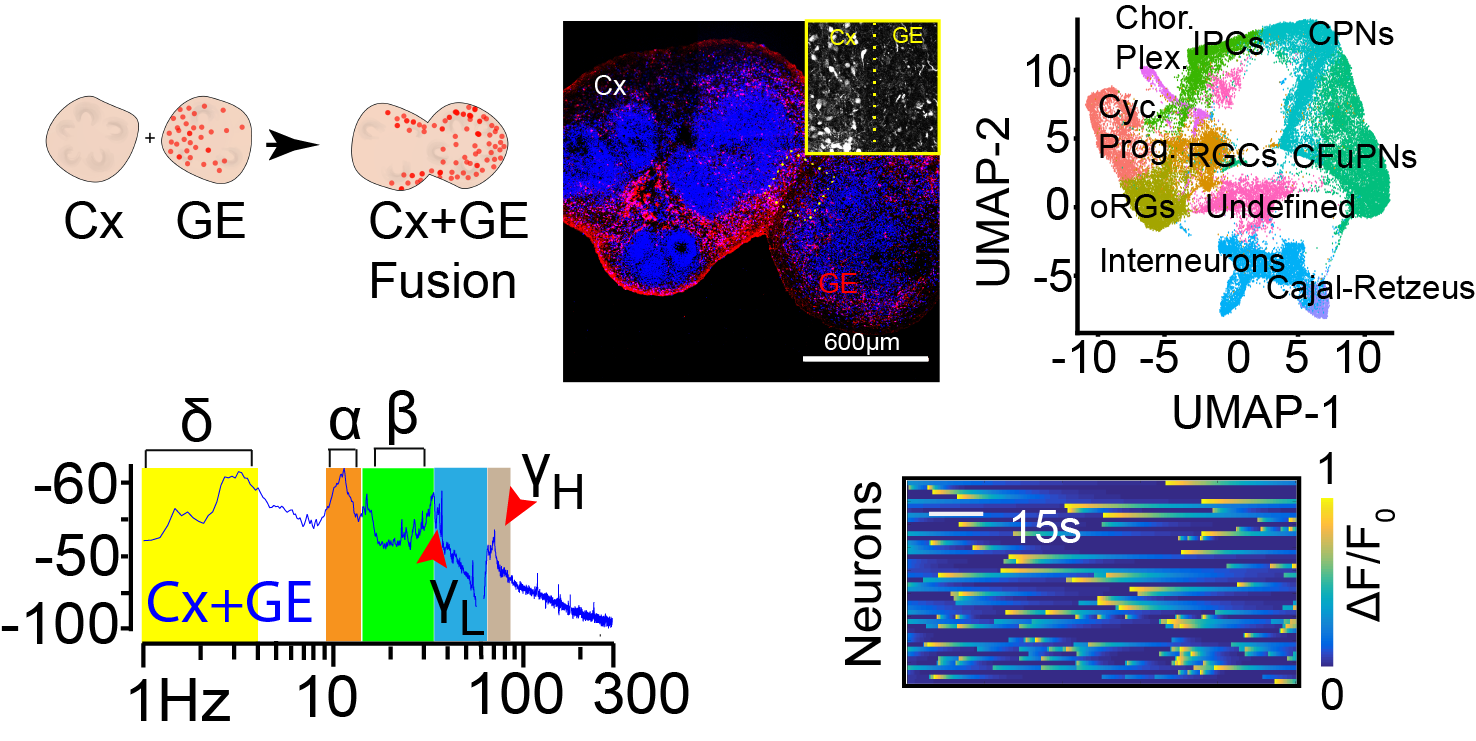
We have developed human stem cell derived brain organoid models that are capable of generating complex physiological activities. These activities include neural oscillations at the same frequencies as are recorded from in vivo human brain and single cell calcium transients that segregate into multiple, distinct, neural networks. To achieve this, we generate “fusion” brain organoids that contain excitatory neurons, inhibitory interneurons, as well as astrocytes. We will now leverage the methodologies we have developed in organoid generation and physiologic monitoring to dive deeper into the mechanisms of normal neural circuit development and how neurological disease perturbs circuit formation and function.
Oscillatory Failure in Autism:
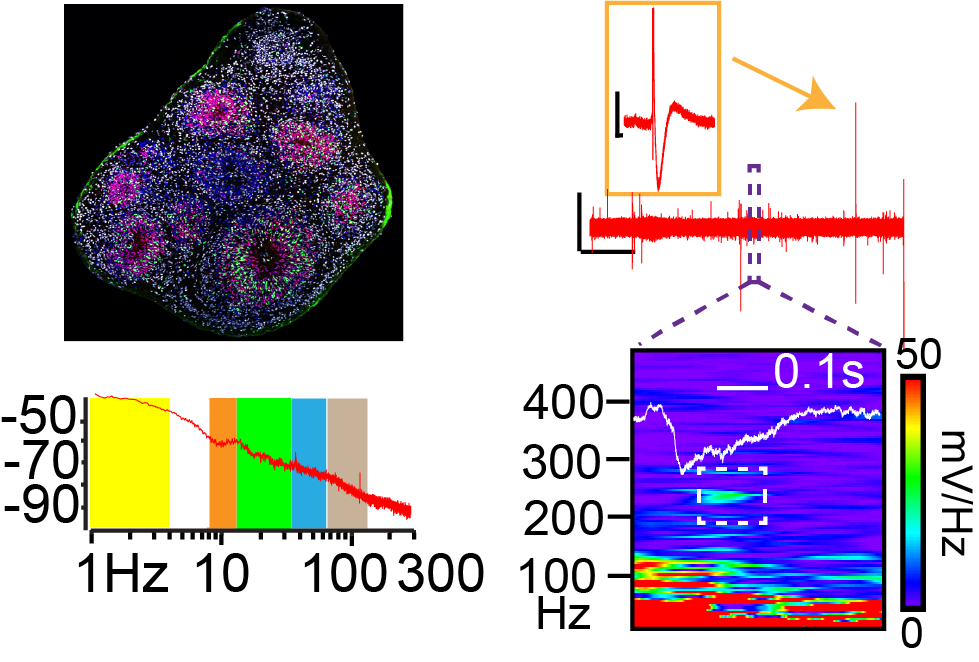
Brain oscillations are believed to be critical for neural computation and can serve as a biomarker of excitatory- inhibitory (E/I) balance in neural circuits. Disruptions in oscillatory activity have been observed in patients with autism spectrum disease (ASD). Indeed, oscillatory dysregulation is thought to be a pathophysiological mechanism linking E/I imbalance with cognitive difficulties in ASD. In particular, a failure to generate gamma oscillations in early life is associated with language, cognitive, and attention deficits characteristic of ASDs. However, uncovering the basic mechanisms linking E/I imbalance to oscillatory changes is difficult given the structural differences between human brain and animal models of ASD, and the challenges of studying basic mechanisms in patients. Uncovering the downstream effects of E/I imbalance on gene and cell expression may also reveal novel therapeutic targets. We recently developed a novel brain organoid platform ideal for modeling the effect of E/I imbalance on downstream cellular plasticity and oscillatory activity. Specifically, we have generated an organoid system where we can consistently generate gamma oscillations.
In preliminary studies, we have shown a loss of gamma oscillatory power in organoids generated from patient induced pluripotent stem cells (iPSCs) harboring autism risk genes. In this project we will use a combination of multiphoton calcium indicator imaging, confocal microscopy, and single cell RNA sequencing to identify the cellular etiology of this change.
An Organoid Model of Hippocampal Circuit Function:
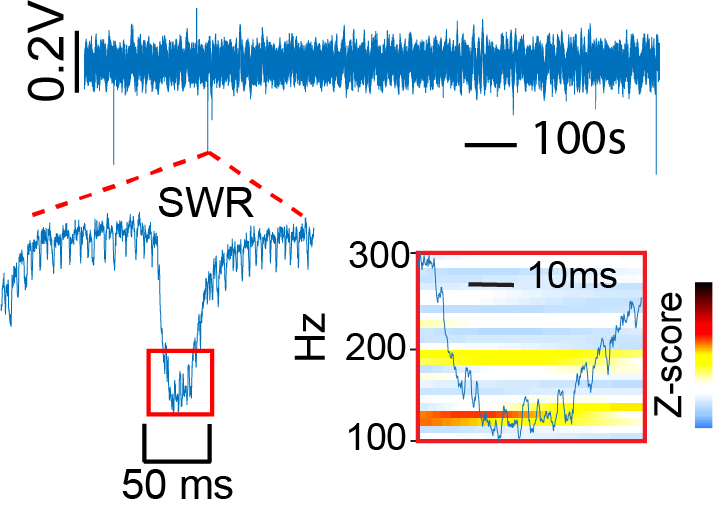
Hippocampal sharp wave ripples (SWRs) are ~100-200Hz oscillations that are thought to be an interneuron-initiatedactivity that is critical to memory consolidation. Interestingly, we frequently observe SWRs in non-mutant hippocampal organoids suggesting that the cellular inputs necessary to generate these can be created in vitro in an organoid. Recently, we also generated hippocampal organoids from patient iPSCs containing a gain of function mutation in the SCN8A gene. As a result of this mutation, these patients typically have a devastating childhood epilepsy known as developmental epileptic encephalopathy 13 (DEE13). Unlike in the case of non-mutant hippocampus, the DEE13 hippocampus so far appears incapable of generating SWRs. In this project we will use chemogenetic, optogenetic, and pharmacological manipulations together with single cell recording, spike sorting, and immunolabeling approaches to identify the mechanisms of SWR generation in hippocampal organoids.
Microglial seeding of human brain organoids: In collaboration with Dr. Jessica Rexach at UCLA, we are initiating a project to seed our brain organoids with Dr. Rexach’s microglia in order to determine the impact of microglia mediated inflammation on neural circuit function. This can be a novel model for studying the role of neural inflammation in a human cell-based system.
Fluorescence Lifetime Imaging (FLIM): This is a unique and powerful imaging technique that we will soon have access to once we acquire our brand-new FLIM capable Leica Stellaris multiphoton scope! FLIM will give us access to a number of powerful techniques such as autofluorescence based live metabolic imaging and multiphoton FLIM-FRET studies. We are eager to utilize this capability in our organoid and stem cell models.
